What Does the Formula of an Ionic Compound Describe
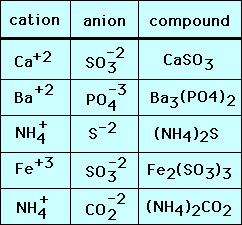
Dipotassium sulfide is incorrect because potassium ion is always K1 and sulfide is always S-2 and therefore the only way to combine them is K2S. ABa2N3 BBa2N2 CBa3N2 DBa3N4.

Writing Ionic Formulas Basic Introduction Youtube
The name and the formula id a molecular compound describe the type and number of atoms in a molecule of the compound.
. What do the name and formula of a molecular compound describe. This is three positive charges and two negative charges. SUMMARY PRINCIPLE OF IONIC COMPOUNDS.
In other words the formula for an ionic compound is an empirical formula. Chemical formulas tell you the identity of the elements in the compound and the relative. Writing the chemical formula for ionic compounds is fairly easy.
In the ionic compound sodium chloride the sodium is 1 and the chlorine is -1. As for ionic compounds it tells us the number of atoms present in one formula unit of the compound. To balance we need two Al3 ions and three O2- ions.
Determine the number of ions in the compound by increasing the number of ions until the net charge is zero. The compound from the other ionic compounds containing the same elements. Aluminium oxide contains Al3 and O2- ions.
Which formula represents an ionic compound and one molecular compound. Eg K2S is potassium sulfide. The ionic compound sodium chloride is made up of sodium cations Na and chloride anions Cl in a 11 ratio.
The ionic bond between ions results from the electrostatic attraction of opposite charges. JAC By Jac Allen Therefore the formulas that represent one ionic compound and one molecular compound is 3 BaCl2 and N2O4. What would be the chemical formula for the ionic compound barium nitride.
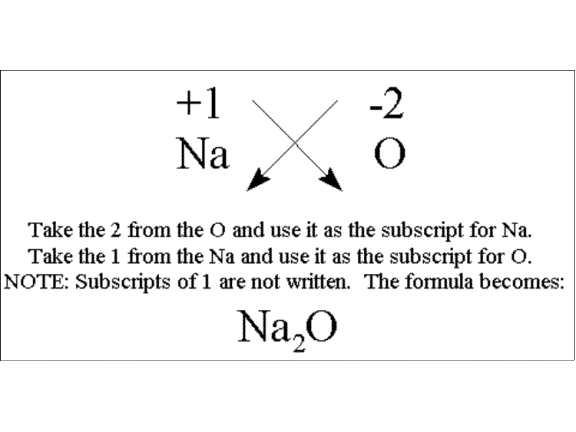
The formula of an ionic compound describes the ratio of the ions in the compound. Beware that this must be the lowest whole ratio of cation to anion. The name of the compound is simply the name of the cation followed by the name of the anion.
K2O one oxide ion for every two potassium ions. The formula simply shows the lowest whole number ratio of the different types of ions NaCl one sodium ion for each chloride ion. So the formula is.
For instanceH2O tells us that there are two hydrogen and one oxygen atom per molecule of water. The formula of an ionic compound describes the ratio of the ions in the compound. What does the formula of an ionic compound describe.
CaF2 one calcium ion for every two fluoride ions. The final formula of sodium fluoride is NaF. The chemical formula of a compound does not indicate the way that elements are joined in the compound.
Write the cation symbol then the anion symbol. The chemical formula for the ionic compound K2S indicates that there isare ____ K ions for every ____ S2- ions. Ionic compounds are also known as electrovalent compounds.
The ratio of the ions in the compound. The chemical bond formed by the transfer of electrons from one atom to another is known as Ionic bond. What is a binary ionic compound.
Cl chloride ion. A compound made from only 2 elements. Na sodium ion.
The formula of an ionic compound represents the lowest whole number ratio of cations to anions it is as simple as that. Formula of Ionic Compounds Cation and Name of Cation Anion and Name of Anion Name of Ionic Compound NaCl. Since Na has an atomic mass of 23 u and Cl has an atomic mass of 355 u the formula mass for NaCl is 23355585 u.
Al 3 aluminum ion. Ionic compounds are neutral compounds made of positive cations and negative anions. What must the name of an ionic compound distinguish.
Determining the Formula of an Ionic Compound A stable ionic compound is electrically neutral where electrons are shared between cations and anions to complete outer electron shells or octets. Ionic Compounds and their Names. Barium ions carry a 2 charge and nitrogen ions carry a 3- charge.
A 1 is written above the symbol for sodium Na and a -1 is written above the symbol for chlorine Cl. Naming simple ionic compounds. BYJUS Online learning Programs For K3 K10 K12 NEET.
An ionic compound is formed by the complete transfer of electrons from a metal to a nonmetal and the resulting ions have achieved an octet. PO 4 3 phosphate ion. You know you have the correct formula for an ionic compound when the positive and negative charges on the ions are the same or cancel each other out.
These compounds contain ions. Ionic Compound Formula and the Principle of Charge Neutrality.
No comments for "What Does the Formula of an Ionic Compound Describe"
Post a Comment